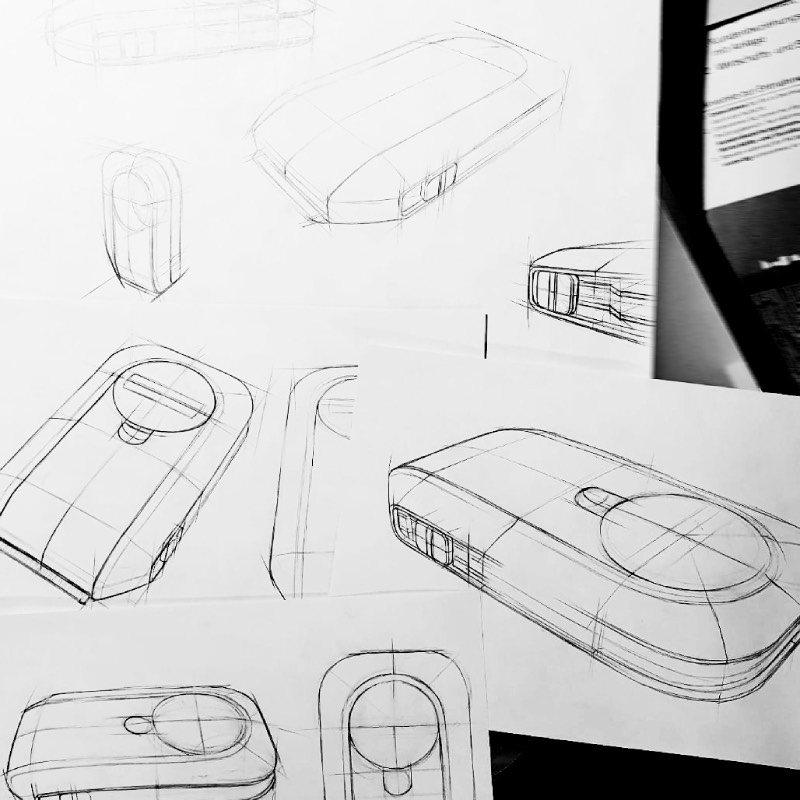
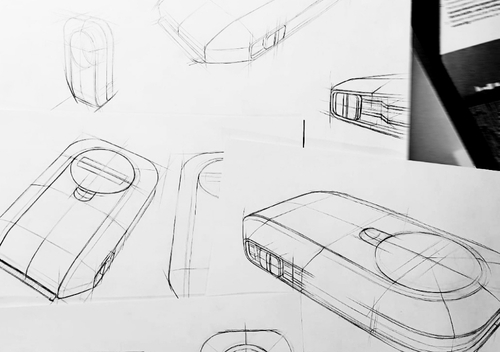
Design as a Service
Early Concept to final design in one stop
Design as a Service brings clarity and direction to complex MedTech ideas from the very first innovation step. With deep insight into clinical workflows, user needs, and technical challenges, we quickly deliver highly realistic early-phase visuals. From the start, every concept integrates critical perspectives – usability, risk analysis, mechanical feasibility, material choices, and regulatory considerations. The result: robust, well-grounded concept visions that help OEMs make informed decisions and launch development processes with confidence.
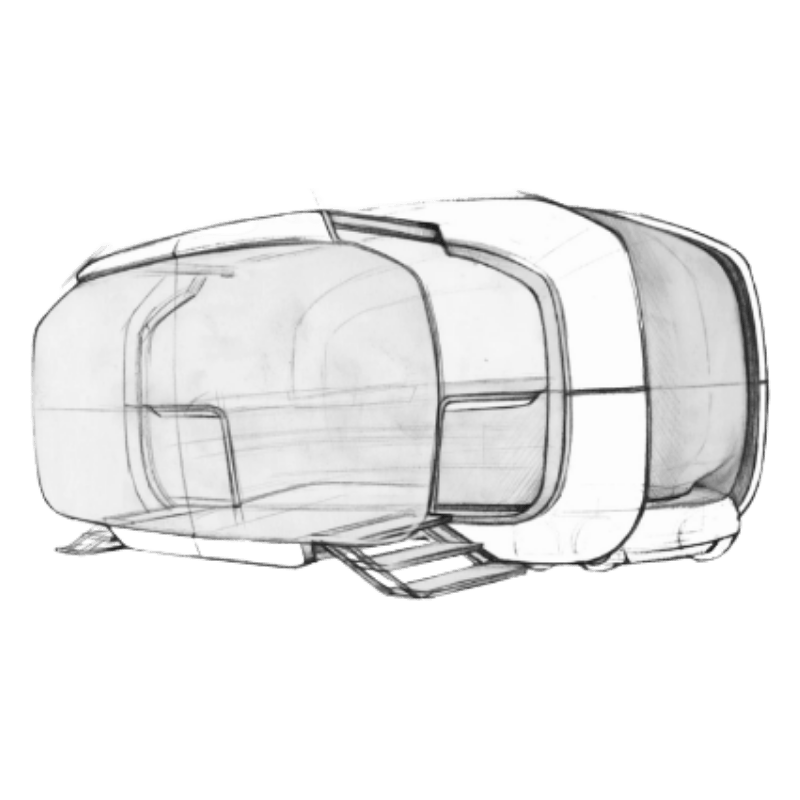
Concept to
Product design
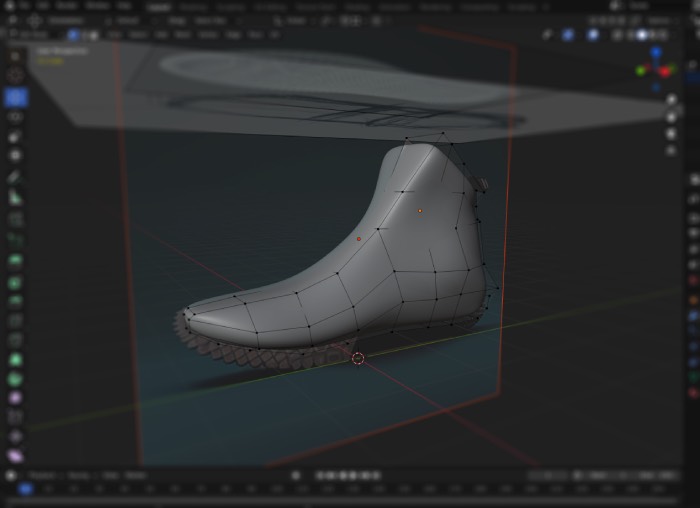
From early ideation visuals
to refined & fully realizable industrial design
Feasibility &
Risk Insight
Material, mechanics, & regulatory considerations
built in early
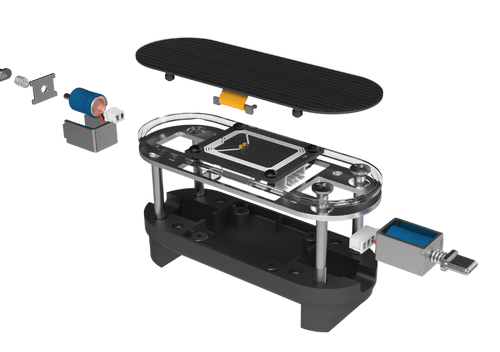
Rapid Prototyping
3D printing & CNC validation
for faster decisions

Human-Centered Design
Creative, intuitive, & ergonomically optimized product usability
Why Integrated Design Defines Product Success
MedTech design is more than aesthetics – it determines usability, compliance, and clinical reliability. Fragmented workflows between design, engineering, and production often cause rework, higher costs, and regulatory delays. Design as a Service eliminates these gaps through unified industrial design and engineering integration.
Siloed Development
Separate design and engineering stages often create usability conflicts. DaaS connects both disciplines from the first sketch, ensuring functional and ergonomic alignment.
Form follows Function
When design trails function, products risk manufacturability, usability, and approval issues. DaaS overcomes this by early validation supported by rapid 3D and CNC prototypes.
Regulatory Delays
Documentation gaps extend CE/FDA approval. Integrated risk management and usability files shorten certification timelines.
Limited Manufacturability
Ignoring production constraints early causes inefficiency. DaaS includes design-for-manufacturing principles from the start.
Advantages and Benefits
Design as a Service combines creative design thinking with technical rigor for OEM product success: from early concepts to validated, production-ready devices.
- Early sketching & ideation provide fast, realistic concept grounding
- Integrated design-to-engineering thinking streamlines later developmentErgonomic, CE/FDA-compliant design ensures global approval readiness
- Rapid prototyping and early validation reduce risk and rework
- Material and interface optimization prevent manufacturing delays
- Scalable support for series production with OEM partners
- Distinctive product design enhances user trust and brand visibility





How You Benefit
Early-Phase Design Efficiency
From initial sketches within a few days to refined industrial design and engineering-ready concepts – a seamless, insight-driven process that reduces complexity, minimizes interfaces, and creates clarity early in the development journey.
Human-Centered & Regulatory-Ready
Ergonomic, intuitive, and aesthetically designed medical device solutions developed from the ground up to meet CE/FDA standards, shortening approval pathways and ensuring compliance from the start.
Rapid Prototyping & Validation
3D printing, CNC machining, and early usability testing accelerate decision-making, reduce costly late-stage changes, and improve confidence in design and function.
Scalable Manufacturing Support
Guidance and resources for series production – leveraging our supplier network or yours – enabling faster global deployment and secure supply chain setup.
Get in Touch
Bring Your MedTech Product Vision to Life
Contact us to learn how Design as a Service transforms MedTech concepts into ergonomic, regulatory-ready products: from first sketch to full-scale production.
- From first sketch to CE/FDA-ready, production-ready devices
- Seamless integration with your existing R&D and supply chain
- Over 30 years of experience in MedTech hardware & engineering
Seamless Integration
into Engineering and Manufacturing Workflows
Design as a Service integrates directly with OEM R&D and engineering teams. Advanced CAD modeling, digital simulation, and interface planning ensure that every design is manufacturable, cleanroom-ready, and regulatory-compliant.
Prototypes undergo usability and ergonomic validation before transfer to scalable production. Materials are selected for radiation compatibility, biocompatibility, and long-term durability. The result: MedTech products that unite functional performance, regulatory reliability, and visual identity in one cohesive design language.
Our Applications
for MedTech Design and Development
Innovative MedTech design drives safer treatments, smoother workflows, and stronger market impact. With Design as a Service, we develop tailored solutions for every clinical field: from radiotherapy and imaging to surgical and patient positioning systems.
- Radiotherapy & Oncology: Industrial design for radiotherapy tables, positioning systems, and treatment accessories with patient comfort and vibration-free precision.
- Imaging & Diagnostics:
- User-driven designs for radiology and diagnostic equipment that combine ergonomic form with radiation hard and MR-safe materials and digital integration.
- Surgical & Operating Room Equipment: Optimized for sterility, intuitive handling, and modular system compatibility within surgical environments.
- Patient Positioning Systems: Human-factors-based solutions for stability, reproducibility, and operator comfort in clinical workflows.
- Prototyping & Product Development: Functional models for usability tests, risk validation, and early regulatory submission readiness.
- Regulatory & Compliance: Design processes aligned with ISO 13485, MDR, and FDA guidelines for traceable, audit-ready documentation.

Radiotherapy & Oncology

Imaging & Diagnostics

Surgical & Operating Room Equipment

Patient Positioning Systems

Prototyping & Product Development

Regulatory & Compliance
Learn more about our design and engineering solutions for OEM partners
Discover how gKteso’s Design as a Service supports global MedTech companies in creating compliant, innovative, and manufacturable devices. From early ideation to validated production, we help OEMs realize new products faster and more reliably.

Learn more about …
Turning complexity into Clarity – with gKteso’s Design as a Service
Schedule an appointment now!

Frequently Asked Questions
What is gKteso Design as a Service?
Design as a Service is our early-phase design support for medical device OEMs – from fast ideation sketches within 1-2 days from your request and realistic concept visuals within 1 week to refined industrial design and engineering-ready concepts. By integrating usability, regulatory, mechanical, and risk insights from the beginning, DaaS enables clearer decisions and smoother downstream development.
Can we use only parts of the service, or is it full-package only?
You can choose. We offer complete product development or integrate seamlessly into your existing R&D process – from single early design sketches to full production-ready solutions.
How do you ensure regulatory compliance (CE/FDA)?
Regulatory requirements are considered from the first concept. We integrate risk management, usability engineering, and documentation early to shorten approval processes and ensure global compliance.
What kind of prototypes can you deliver?
We provide high-fidelity prototypes using 3D printing, CNC machining, and functional models for demo mock-ups, usability tests, ergonomic validation, and mechanical integration.
How does gKteso support the transition to manufacturing?
gKteso offers a fully integrated, end-to-end pathway under one roof – from early design and engineering to prototyping and manufacturing preparation. With all disciplines in-house, handover gaps disappear, decisions move faster, and the transition into scalable, CE/FDA-ready production becomes seamless.
Explore Our Contract Manufacturing Capabilities

Contract Manufacturing
Developed for medical technology innovators and OEMs, our Contract Manufacturing Services turn complex device concepts into certified, market-ready products. From rapid prototyping to validated large-scale production, we ensure regulatory compliance, precision, and supply chain reliability

Customized MLC
Tailored for OEMs, research centers, and novel radiotherapy platforms, our customized MLCs are co-developed to meet unique geometric, electronic, or integration needs. We support you from CAD concept to validated hardware, whether for FLASH, MRI-based therapy, compact systems, or proton beamlines.

Customized OEM QA tools
Customized OEM QA Phantoms and Water Phantom Transport Units from gKteso provide tailored solutions for commissioning, routine QA, imaging calibration, and OEM system integration. With reproducible precision, MDR/FDA compliance, MR compatible options and scalable manufacturing, they ensure reliability in clinical and research environments worldwide.